diff --git a/source/_posts/DeePMD_13_12_2024.md b/source/_posts/DeePMD_13_12_2024.md
index ee12c9de..1159dc1c 100644
--- a/source/_posts/DeePMD_13_12_2024.md
+++ b/source/_posts/DeePMD_13_12_2024.md
@@ -5,7 +5,7 @@ categories:
- DeePMD-kit
---
-Recently, Associate Professor Yang Xu from the School of Science at Shenyang Aerospace University, in cooperation with Professor Du Fei and Professor Zeng Yi from Jilin University and other scholars, conducted an in-depth study on the cubic phase $K_{3}SbS_{4}$ solid-state electrolyte based on the DeePMD method. The related research results were published in the journal "Chemistry of Materials" under the title "Cl-Doped Cubic $K_{3}SbS_{4}$ as a Solid-State Electrolyte for K-Ion Batteries with Ultrafast Ionic Conductivity" (DOI: 10.1021/acs.chemmater.4c02575).
+Recently, Associate Professor Xu Yang from the School of Science at Shenyang Aerospace University, in cooperation with Professor Fei Du and Professor Yi Zeng from Jilin University and other scholars, conducted an in-depth study on the cubic phase $K_{3}SbS_{4}$ solid-state electrolyte based on the DeePMD method. The related research results were published in the journal "Chemistry of Materials" under the title "Cl-Doped Cubic $K_{3}SbS_{4}$ as a Solid-State Electrolyte for K-Ion Batteries with Ultrafast Ionic Conductivity" (DOI: 10.1021/acs.chemmater.4c02575).
@@ -34,15 +34,19 @@ We also tested the size of the model and the simulation time. In each set of con
Further, we used DeePMD to conduct a systematic study of the system. The doping of Cl at the S site introduced K vacancies, which promoted the vacancy diffusion of neighboring K positions and thus significantly increased the transmission rate. By analyzing the motion trajectory of potassium ions (see Figure 3), it can be clearly observed that the K ions change from thermal motion near the equilibrium position to diffusion in three-dimensional directions. The trajectory analysis results show that a small amount of doping can increase the ionic conductivity to 14.8 mS/cm, and the corresponding activation energy is only 0.12 eV.
+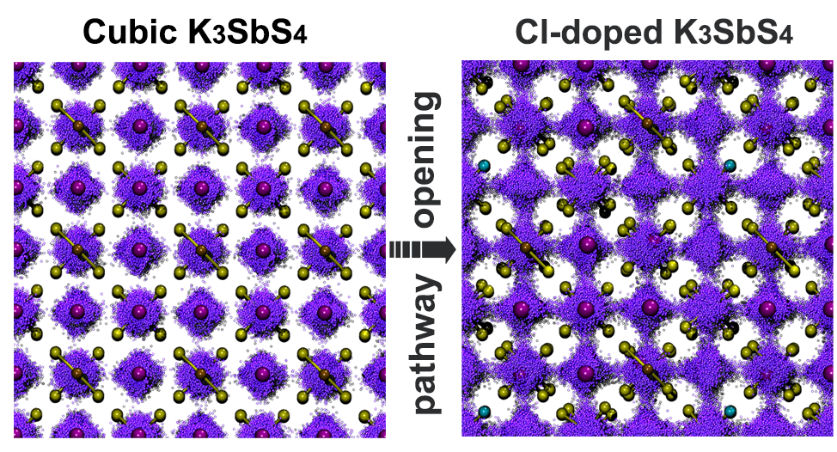 +
+*Figure 3: Influence of Doping on the Trajectory of Potassium Ions*
+
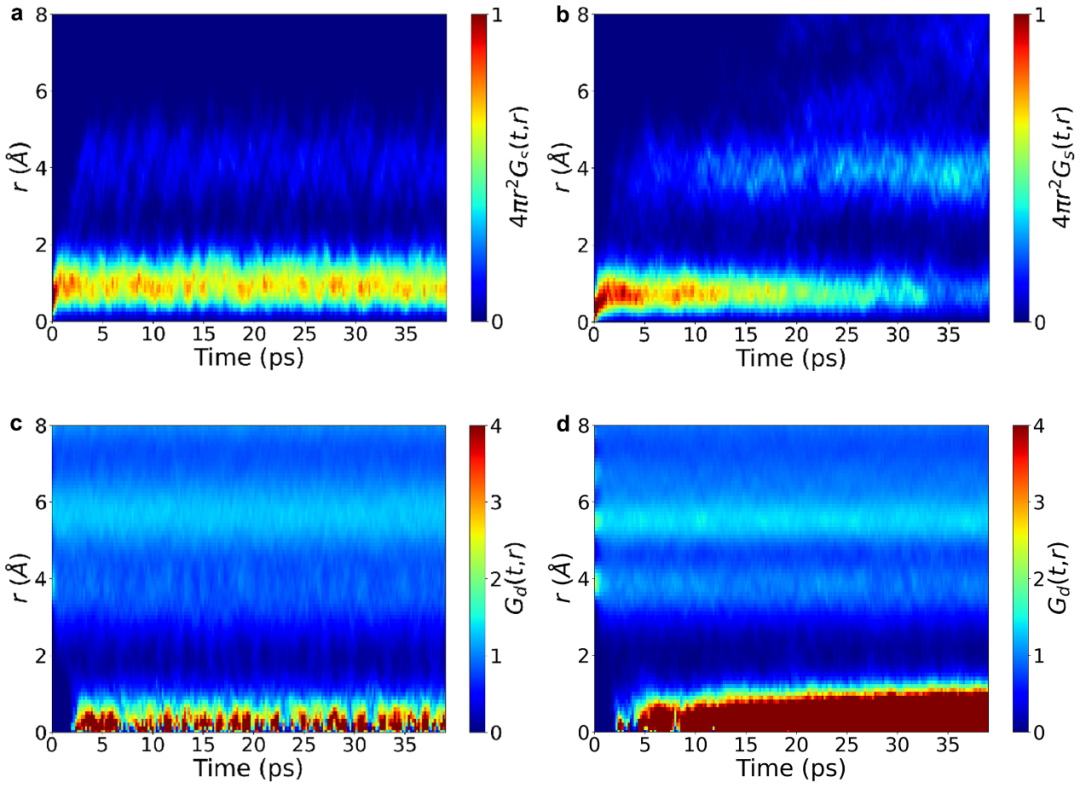
Finally, we introduced the Van Hove correlation function.2 It can be seen that for the undoped system (Figure 4a, c), the correlation function parts $G_{s}(t,r)$ of the same particles at different times and the correlation function parts $G_{d}(t,r)$ of different particles are relatively stable, which indicates that the particles do not migrate. In contrast, for the doped system, whether it is the disappearance of $G_{s}(t,r)$ at $r = 1\ Å$ or the gradual accumulation of $G_{d}(t, r)$ with time, both show the typical characteristics of highly correlated jumps of particles.
-## Summary
+
+
+*Figure 3: Influence of Doping on the Trajectory of Potassium Ions*
+
Finally, we introduced the Van Hove correlation function.2 It can be seen that for the undoped system (Figure 4a, c), the correlation function parts $G_{s}(t,r)$ of the same particles at different times and the correlation function parts $G_{d}(t,r)$ of different particles are relatively stable, which indicates that the particles do not migrate. In contrast, for the doped system, whether it is the disappearance of $G_{s}(t,r)$ at $r = 1\ Å$ or the gradual accumulation of $G_{d}(t, r)$ with time, both show the typical characteristics of highly correlated jumps of particles.
-## Summary
+ -We used a neural network to train the potential model of the cube $K_{3}SbS_{4}$, and systematically studied the impact of model scale and simulation time on quantum fluctuations. On this basis, we analyzed the impact of chlorine doping on the ion diffusion coefficient through the DeePMD method. The research results show that vacancies play a crucial role in the ion diffusion process, and even a small number of vacancies can transform this material from an ion insulator to an efficient ion conductor. The analysis of the Van Hove correlation function further reveals that potassium vacancies significantly increase the jump frequency of potassium ions. At 300 K, the ionic conductivity of this material is as high as 14.8 S/cm, and its diffusion energy barrier is only 0.12 eV. Therefore, the doped cube $K_{3}SbS_{4}$ is regarded as a promising potassium-ion solid-state electrolyte.
+*Figure 4: Van Hove Correlation Function of the Undoped and Doped Systems*
-
-We used a neural network to train the potential model of the cube $K_{3}SbS_{4}$, and systematically studied the impact of model scale and simulation time on quantum fluctuations. On this basis, we analyzed the impact of chlorine doping on the ion diffusion coefficient through the DeePMD method. The research results show that vacancies play a crucial role in the ion diffusion process, and even a small number of vacancies can transform this material from an ion insulator to an efficient ion conductor. The analysis of the Van Hove correlation function further reveals that potassium vacancies significantly increase the jump frequency of potassium ions. At 300 K, the ionic conductivity of this material is as high as 14.8 S/cm, and its diffusion energy barrier is only 0.12 eV. Therefore, the doped cube $K_{3}SbS_{4}$ is regarded as a promising potassium-ion solid-state electrolyte.
+*Figure 4: Van Hove Correlation Function of the Undoped and Doped Systems*
- +## Summary
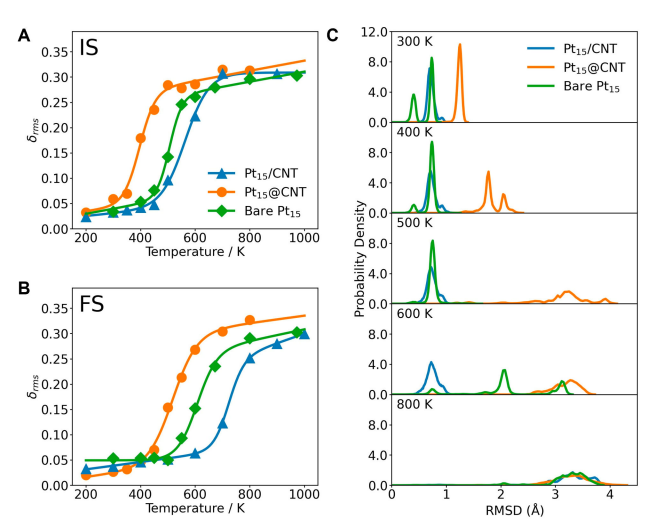
-*Figure 4 Phase transition behaviors of Pt₁₅@CNT, Pt₁₅/CNT, and bare Pt₁₅ in the initial state (IS, A) and final state (FS, B). The calculated values are represented by points, and the corresponding fitted curves are represented by lines. (C) Probability density of the RMSD of the Pt atom positions of Pt₁₅@CNT, bare Pt₁₅, and Pt₁₅/CNT in the final state (FS) at different temperatures. The RMSD of the Pt atom positions is calculated from the MD trajectory relative to the first frame.*
+We used a neural network to train the potential model of the cube $K_{3}SbS_{4}$, and systematically studied the impact of model scale and simulation time on quantum fluctuations. On this basis, we analyzed the impact of chlorine doping on the ion diffusion coefficient through the DeePMD method. The research results show that vacancies play a crucial role in the ion diffusion process, and even a small number of vacancies can transform this material from an ion insulator to an efficient ion conductor. The analysis of the Van Hove correlation function further reveals that potassium vacancies significantly increase the jump frequency of potassium ions. At 300 K, the ionic conductivity of this material is as high as 14.8 S/cm, and its diffusion energy barrier is only 0.12 eV. Therefore, the doped cube $K_{3}SbS_{4}$ is regarded as a promising potassium-ion solid-state electrolyte.
## Outlook
+## Summary
-*Figure 4 Phase transition behaviors of Pt₁₅@CNT, Pt₁₅/CNT, and bare Pt₁₅ in the initial state (IS, A) and final state (FS, B). The calculated values are represented by points, and the corresponding fitted curves are represented by lines. (C) Probability density of the RMSD of the Pt atom positions of Pt₁₅@CNT, bare Pt₁₅, and Pt₁₅/CNT in the final state (FS) at different temperatures. The RMSD of the Pt atom positions is calculated from the MD trajectory relative to the first frame.*
+We used a neural network to train the potential model of the cube $K_{3}SbS_{4}$, and systematically studied the impact of model scale and simulation time on quantum fluctuations. On this basis, we analyzed the impact of chlorine doping on the ion diffusion coefficient through the DeePMD method. The research results show that vacancies play a crucial role in the ion diffusion process, and even a small number of vacancies can transform this material from an ion insulator to an efficient ion conductor. The analysis of the Van Hove correlation function further reveals that potassium vacancies significantly increase the jump frequency of potassium ions. At 300 K, the ionic conductivity of this material is as high as 14.8 S/cm, and its diffusion energy barrier is only 0.12 eV. Therefore, the doped cube $K_{3}SbS_{4}$ is regarded as a promising potassium-ion solid-state electrolyte.
## Outlook



