diff --git a/source/_posts/DeePMD_13_12_2024.md b/source/_posts/DeePMD_13_12_2024.md
index 6bfe5c2f..991d35c9 100644
--- a/source/_posts/DeePMD_13_12_2024.md
+++ b/source/_posts/DeePMD_13_12_2024.md
@@ -5,7 +5,7 @@ categories:
- DeePMD-kit
---
-Recently, Associate Professor Xu Yang from the School of Science at Shenyang Aerospace University, in cooperation with Professor Fei Du and Professor Yi Zeng from Jilin University and other scholars, conducted an in-depth study on the cubic phase $K_{3}SbS_{4}$ solid-state electrolyte based on the DeePMD method. The related research results were published in the journal "Chemistry of Materials" under the title "Cl-Doped Cubic K3SbS4 as a Solid-State Electrolyte for K-Ion Batteries with Ultrafast Ionic Conductivity" (DOI: 10.1021/acs.chemmater.4c02575).
+Recently, Associate Professor Xu Yang from the School of Science at Shenyang Aerospace University, in cooperation with Professor Fei Du and Professor Yi Zeng from Jilin University and other scholars, conducted an in-depth study on the cubic phase K3SbS4 solid-state electrolyte based on the DeePMD method. The related research results were published in the journal "Chemistry of Materials" under the title "Cl-Doped Cubic K3SbS4 as a Solid-State Electrolyte for K-Ion Batteries with Ultrafast Ionic Conductivity" (DOI: 10.1021/acs.chemmater.4c02575).
@@ -14,9 +14,9 @@ Recently, Associate Professor Xu Yang from the School of Science at Shenyang Aer
The development of efficient solid-state electrolytes (SSEs) is crucial for the progress of energy storage technologies, especially in the context of potassium-ion batteries, and is of great significance for improving the safety and stability of batteries. The relatively large radius of potassium ions makes it difficult to transplant many lithium-ion solid-state electrolytes into potassium-ion batteries.
-The cubic phase $A_{3}MS_{4}~(A = Na, K; M = Sb, P)$ materials have an open three-dimensional framework structure, which is beneficial for the rapid transmission of ions. However, due to the full occupation of the A site by alkali metals and the effect of electrostatic repulsion, it is difficult for them to diffuse to adjacent A sites, so the unmodified materials usually have a very low ionic conductivity. Complex experimental explorations are often time-consuming and laborious, and the technical obstacles in phase synthesis limit the rapid development of potassium solid-state electrolytes.
+The cubic phase A3MS4 (A = Na, K; M = Sb, P) materials have an open three-dimensional framework structure, which is beneficial for the rapid transmission of ions. However, due to the full occupation of the A site by alkali metals and the effect of electrostatic repulsion, it is difficult for them to diffuse to adjacent A sites, so the unmodified materials usually have a very low ionic conductivity. Complex experimental explorations are often time-consuming and laborious, and the technical obstacles in phase synthesis limit the rapid development of potassium solid-state electrolytes.
-Molecular dynamics simulation has become an important tool for understanding the properties and mechanisms of solid-state electrolytes. However, the first-principles molecular dynamics simulation (AIMD) is slow in calculation because it needs to continuously solve the Schrödinger equation, and the atom system that can be simulated usually does not exceed 300 - 500 atoms. Due to insufficient sampling, the results of each simulation for the same system at the same temperature may vary, as has been shown in our previous article.1 This study focuses on the impact of Cl doping on the potassium-ion diffusion rate in the cubic phase $K_{3}SbS_{4}$ using the DeePMD method. The doped material can achieve an ionic conductivity of 14.8 mS/cm at a temperature of 300 K. Thanks to the relatively fast calculation speed, we also evaluated the impact of sampling time and model size on the reproducibility of simulation results. The results show that a simulation of a 3400-atom system for 500 ps can ensure good reproducibility.
+Molecular dynamics simulation has become an important tool for understanding the properties and mechanisms of solid-state electrolytes. However, the first-principles molecular dynamics simulation (AIMD) is slow in calculation because it needs to continuously solve the Schrödinger equation, and the atom system that can be simulated usually does not exceed 300 - 500 atoms. Due to insufficient sampling, the results of each simulation for the same system at the same temperature may vary, as has been shown in our previous article.1 This study focuses on the impact of Cl doping on the potassium-ion diffusion rate in the cubic phase K3SbS4 using the DeePMD method. The doped material can achieve an ionic conductivity of 14.8 mS/cm at a temperature of 300 K. Thanks to the relatively fast calculation speed, we also evaluated the impact of sampling time and model size on the reproducibility of simulation results. The results show that a simulation of a 3400-atom system for 500 ps can ensure good reproducibility.
## Research Results
@@ -38,7 +38,7 @@ Further, we used DeePMD to conduct a systematic study of the system. The doping
*Figure 3: Influence of Doping on the Trajectory of Potassium Ions*
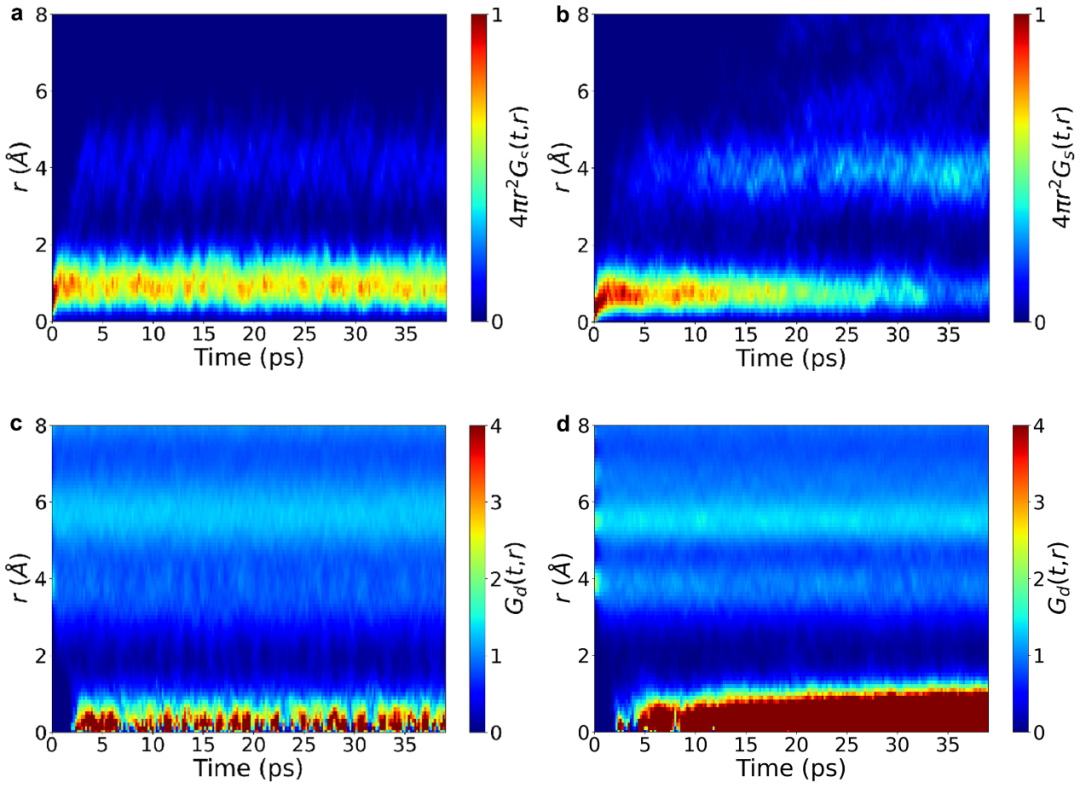
-Finally, we introduced the Van Hove correlation function.2 It can be seen that for the undoped system (Figure 4a, c), the correlation function parts $G_{s}(t,r)$ of the same particles at different times and the correlation function parts $G_{d}(t,r)$ of different particles are relatively stable, which indicates that the particles do not migrate. In contrast, for the doped system, whether it is the disappearance of $G_{s}(t,r)$ at $r = 1\ Å$ or the gradual accumulation of $G_{d}(t, r)$ with time, both show the typical characteristics of highly correlated jumps of particles.
+Finally, we introduced the Van Hove correlation function.2 It can be seen that for the undoped system (Figure 4a, c), the correlation function parts Gs(t,r) of the same particles at different times and the correlation function parts Gd(t,r) of different particles are relatively stable, which indicates that the particles do not migrate. In contrast, for the doped system, whether it is the disappearance of Gs(t,r) in r = 1 Å or the gradual accumulation of Gd(t,r) with time, both show the typical characteristics of highly correlated jumps of particles.
 @@ -46,7 +46,7 @@ Finally, we introduced the Van Hove correlation function.2 It can be seen that f
## Summary
-We used a neural network to train the potential model of the cube $K_{3}SbS_{4}$, and systematically studied the impact of model scale and simulation time on quantum fluctuations. On this basis, we analyzed the impact of chlorine doping on the ion diffusion coefficient through the DeePMD method. The research results show that vacancies play a crucial role in the ion diffusion process, and even a small number of vacancies can transform this material from an ion insulator to an efficient ion conductor. The analysis of the Van Hove correlation function further reveals that potassium vacancies significantly increase the jump frequency of potassium ions. At 300 K, the ionic conductivity of this material is as high as 14.8 S/cm, and its diffusion energy barrier is only 0.12 eV. Therefore, the doped cube $K_{3}SbS_{4}$ is regarded as a promising potassium-ion solid-state electrolyte.
+We used a neural network to train the potential model of the cube K3SbS4, and systematically studied the impact of model scale and simulation time on quantum fluctuations. On this basis, we analyzed the impact of chlorine doping on the ion diffusion coefficient through the DeePMD method. The research results show that vacancies play a crucial role in the ion diffusion process, and even a small number of vacancies can transform this material from an ion insulator to an efficient ion conductor. The analysis of the Van Hove correlation function further reveals that potassium vacancies significantly increase the jump frequency of potassium ions. At 300 K, the ionic conductivity of this material is as high as 14.8 S/cm, and its diffusion energy barrier is only 0.12 eV. Therefore, the doped cube K3SbS4 is regarded as a promising potassium-ion solid-state electrolyte.
## Outlook
@@ -46,7 +46,7 @@ Finally, we introduced the Van Hove correlation function.2 It can be seen that f
## Summary
-We used a neural network to train the potential model of the cube $K_{3}SbS_{4}$, and systematically studied the impact of model scale and simulation time on quantum fluctuations. On this basis, we analyzed the impact of chlorine doping on the ion diffusion coefficient through the DeePMD method. The research results show that vacancies play a crucial role in the ion diffusion process, and even a small number of vacancies can transform this material from an ion insulator to an efficient ion conductor. The analysis of the Van Hove correlation function further reveals that potassium vacancies significantly increase the jump frequency of potassium ions. At 300 K, the ionic conductivity of this material is as high as 14.8 S/cm, and its diffusion energy barrier is only 0.12 eV. Therefore, the doped cube $K_{3}SbS_{4}$ is regarded as a promising potassium-ion solid-state electrolyte.
+We used a neural network to train the potential model of the cube K3SbS4, and systematically studied the impact of model scale and simulation time on quantum fluctuations. On this basis, we analyzed the impact of chlorine doping on the ion diffusion coefficient through the DeePMD method. The research results show that vacancies play a crucial role in the ion diffusion process, and even a small number of vacancies can transform this material from an ion insulator to an efficient ion conductor. The analysis of the Van Hove correlation function further reveals that potassium vacancies significantly increase the jump frequency of potassium ions. At 300 K, the ionic conductivity of this material is as high as 14.8 S/cm, and its diffusion energy barrier is only 0.12 eV. Therefore, the doped cube K3SbS4 is regarded as a promising potassium-ion solid-state electrolyte.
## Outlook

