diff --git a/source/_posts/DeePMD_03_06_2025.md b/source/_posts/DeePMD_03_06_2025.md
new file mode 100644
index 0000000..beef3fb
--- /dev/null
+++ b/source/_posts/DeePMD_03_06_2025.md
@@ -0,0 +1,59 @@
+---
+title: "What Can DP Do too? | DP Aids in the Study of Microscopic Reaction Mechanisms of Vanadium Removal from Crude Titanium Tetrachloride by Aluminum"
+date: 2025-06-03
+categories:
+- DeePMD
+---
+
+Recently, the research team of Professor Chen Xiumin from the National Engineering Research Center for Vacuum Metallurgy, Kunming University of Science and Technology, in collaboration with DeepSeek, has achieved research on the microscopic reaction mechanism of vanadium removal from crude titanium tetrachloride by aluminum addition through a new method of artificial intelligence-driven scientific research (AI for Science). This study utilized the Deep Potential Molecular Dynamics (DPMD) simulation method to efficiently explore the reaction mechanism of vanadium removal by aluminum addition at the nanosecond time scale and the spatial scale of tens of thousands of atoms. Theoretical simulation analysis and experimental research show that the vanadium removal reaction is a synergistic mechanism of reduction and complexation reactions. In the Al-Cl₂-TiCl₄-VOCl₃ system, the reduction process forms polynuclear complexes with aluminum, titanium, and vanadium as central atoms bridged by Cl and O atoms. These polynuclear complexes, catalyzed by AlCl₃, convert VOCl₃ into VOCl₂ and VCl₃ through the exchange and transfer of Cl and O atoms in two reaction pathways. In this study, DPMD provides a new means to understand specific reactions from a microscopic perspective. The study of this reaction mechanism not only helps with the recycling and utilization of vanadium resources but also provides a theoretical basis and innovative ideas for the optimization and improvement of vanadium removal reagents.
+
+Paper Link:https://j1q.cn/zbEVYBDj
+
+
+
+## Research Background
+
+Titanium tetrachloride (TiCl₄) is a key process raw material for preparing sponge titanium and titanium dioxide. However, vanadium in crude TiCl₄ prepared by carbothermal chlorination of high-titanium slag exists in the form of VOCl₃, which is miscible with TiCl₄ and has a low separation coefficient, making it difficult to remove by physical methods. This leads to degraded performance of the prepared sponge titanium and poor performance of titanium dioxide pigments. Vanadium itself is a critical metal element with "rare", "green", and "energy" functions, finding applications in steel industry, energy storage, chemical industry, aerospace, national defense, and environmental governance. The essence of vanadium removal from crude titanium tetrachloride is the recovery of titanium resources through separation and purification. Therefore, effectively removing vanadium from crude titanium tetrachloride is of great significance for the subsequent recovery of vanadium resources.
+
+Aluminum powder vanadium removal is one of the effective processes for removing vanadium from crude titanium tetrachloride due to its easy separation of residues, small environmental impact, and ability to obtain high-quality refined TiCl₄. However, aluminum powder vanadium removal also has problems such as batch operation. Therefore, clarifying the reaction mechanism of vanadium removal by aluminum addition is of great significance for process optimization. However, due to the corrosiveness and volatility of titanium tetrachloride and the fast reaction rate of aluminum vanadium removal, it is difficult to study the reaction mechanism of vanadium removal by experimental methods. Current mechanism research is limited to the thermodynamic and experimental stages. With the development of artificial intelligence algorithms, the research and development model of exploring chemical reaction mechanisms from the microscale and providing theoretical guidance for the improvement of production processes has been widely developed in many aspects of the chemical industry. The Deep Potential Molecular Dynamics (DPMD) simulation method is a classic case where artificial intelligence algorithms successfully combine first-principles calculations with traditional molecular dynamics simulation methods. This method uses training data obtained from first-principles (AIMD) to train a deep potential (DP) model through a deep neural network and uses the LAMMPS interface supported by DeePMD-kit to call the deep potential (DP) model to run classical molecular dynamics simulations. This not only makes up for the shortcomings of first-principles dynamics simulations in time and space scales but also provides accuracy comparable to AIMD and efficiency similar to empirical potentials. This method is playing an increasingly important role in reaction mechanism research. Therefore, this paper introduces the DPMD simulation method to study the microscopic change process of chemical reactions in the aluminum powder vanadium removal system from the atomic and molecular scales and combines thermodynamic theoretical calculations and experiments to verify the reaction mechanism, so as to provide a theoretical basis for the development of more excellent vanadium removal methods.
+
+This study first carried out a thermodynamic analysis of the possible chemical reactions in the process of vanadium removal by aluminum powder. Secondly, the potential function model obtained based on first-principles accuracy data was used for DPMD simulation to explore the reaction path and microscopic mechanism of VOCl₃ removal by aluminum powder. Finally, experiments of vanadium removal by aluminum addition were carried out through steps such as reaction, distillation, centrifugation, filtration, and drying to obtain residues, which were characterized by XPS, XRD, SEM, EDS, and other means to verify the theoretical calculation results. The study shows that vanadium removal by aluminum powder is achieved through two paths of converting VOCl₃ into VOCl₂ and VCl₃, providing help for industrial recovery of vanadium resources and a theoretical basis for optimizing vanadium removal reagents.
+
+## Microscopic Reaction Mechanism of Vanadium Removal by Aluminum
+
+
+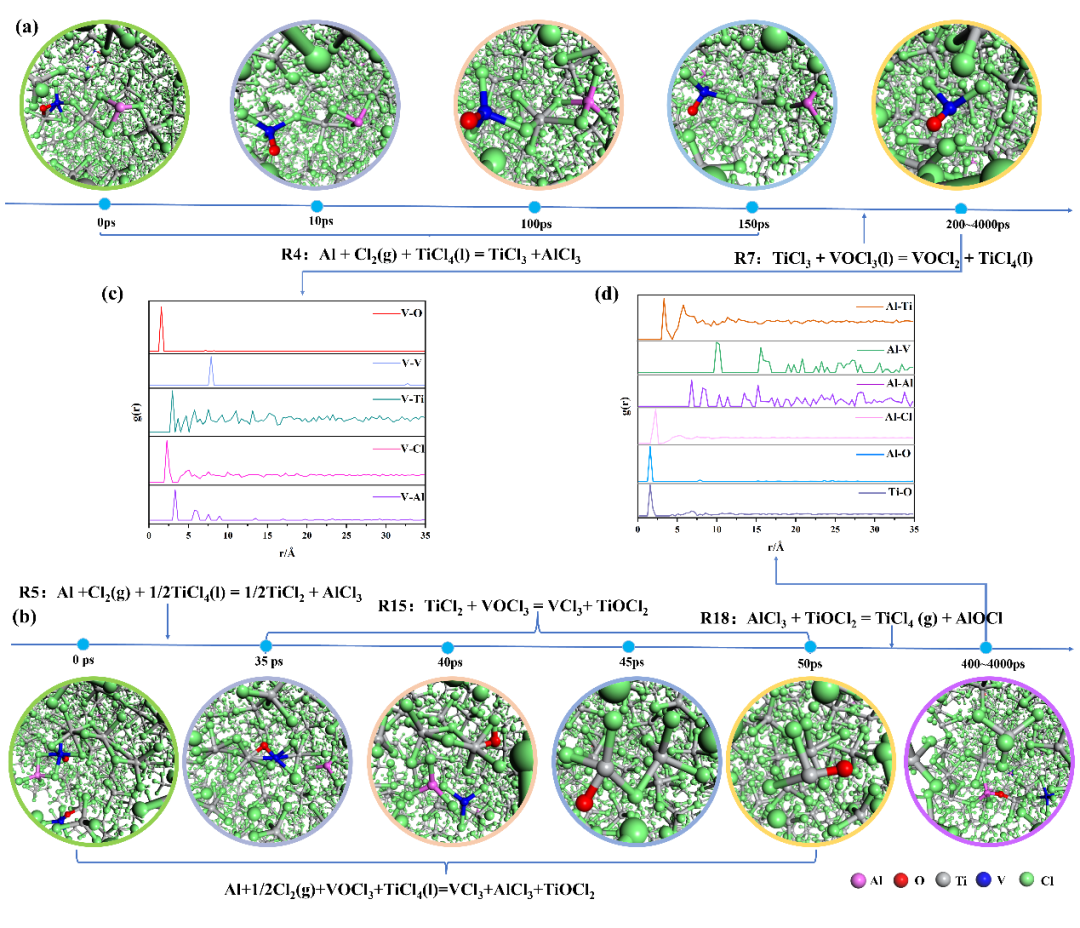 +
+*Fig. 1 (a), (b) the microstructure evolution images of two reaction pathways of VOCl3 in the 413K Al-Cl2-TiCl4-VOCl3 system from DPMD; (c) Fig. (a) RDF image of different atoms around V at 200 ps; (d) Fig. (b) RDF image of different atoms around Al and between Ti-O at 400 ps*
+
+
+Figure 1(b) shows the reaction pathway of VOCl₃ transforming into VCl₃ in the Al-Cl₂-TiCl₄-VOCl₃ system. Initially, at 0 ps, a polynuclear complex of AlCl₃-VOCl₃·3TiCl₄ is formed through multiple Cl atoms. As the simulation time reaches 35 ps, AlCl₃ separates from the polynuclear complex, and the polynuclear complex is bridged by Ti-O bonds, forming a polynuclear complex similar to the Al-TiCl₄-VOCl₃ system. When the DPMD simulation reaches 40 ps, it is found that O in VOCl₃ separates and converts into VCl₃, and Cl and Ti atoms in the binuclear complex composed of two titanium chlorides combine with O. AlCl₃ also appears around VCl₃, indicating that AlCl₃ catalyzes titanium to reduce VOCl₃ to VCl₃. In the DPMD simulation at 45 ps, a TiCl₂O-TiCl₄ polynuclear complex bound by one Cl atom is found, which gradually converts to TiCl₂O by 50 ps. As the DPMD simulation reaches 400 ps, a polynuclear complex connected by Al-O-Ti bonds appears, which is the same as that in the Al-TiCl₄-VOCl₃ system at 750 ps, with subsequent reactions between TiCl₂O and AlCl₃.
+
+According to the DPMD simulation results of the Al-Cl₂-TiCl₄-VOCl₃ system, the vanadium removal mechanism is a synergistic reaction mechanism of reduction-complexation. It mainly forms polynuclear complexes with aluminum chloride, titanium chloride, and vanadyl chloride as central atoms bridged by Cl and O atoms in the reduction reaction. The polynuclear complexes undergo charge transfer and chemical reactions through the exchange and transfer of Cl and O atoms catalyzed by aluminum chloride (AlCl₃), removing VOCl₃ from crude titanium tetrachloride through two reaction pathways: one is that low-valent titanium (TiCl₃) converts VOCl₃ into insoluble VOCl₂; the other is that low-valent titanium (TiCl₂) converts VOCl₃ into insoluble VCl₃, with the generation of TiCl₂O. However, the finally generated AlCl₃ in the reaction system will convert TiCl₂O into TiCl₄, in which VOCl₃ is more easily converted into VCl₃.
+
+## Experimental Verification
+
+
+
+
+*Fig. 1 (a), (b) the microstructure evolution images of two reaction pathways of VOCl3 in the 413K Al-Cl2-TiCl4-VOCl3 system from DPMD; (c) Fig. (a) RDF image of different atoms around V at 200 ps; (d) Fig. (b) RDF image of different atoms around Al and between Ti-O at 400 ps*
+
+
+Figure 1(b) shows the reaction pathway of VOCl₃ transforming into VCl₃ in the Al-Cl₂-TiCl₄-VOCl₃ system. Initially, at 0 ps, a polynuclear complex of AlCl₃-VOCl₃·3TiCl₄ is formed through multiple Cl atoms. As the simulation time reaches 35 ps, AlCl₃ separates from the polynuclear complex, and the polynuclear complex is bridged by Ti-O bonds, forming a polynuclear complex similar to the Al-TiCl₄-VOCl₃ system. When the DPMD simulation reaches 40 ps, it is found that O in VOCl₃ separates and converts into VCl₃, and Cl and Ti atoms in the binuclear complex composed of two titanium chlorides combine with O. AlCl₃ also appears around VCl₃, indicating that AlCl₃ catalyzes titanium to reduce VOCl₃ to VCl₃. In the DPMD simulation at 45 ps, a TiCl₂O-TiCl₄ polynuclear complex bound by one Cl atom is found, which gradually converts to TiCl₂O by 50 ps. As the DPMD simulation reaches 400 ps, a polynuclear complex connected by Al-O-Ti bonds appears, which is the same as that in the Al-TiCl₄-VOCl₃ system at 750 ps, with subsequent reactions between TiCl₂O and AlCl₃.
+
+According to the DPMD simulation results of the Al-Cl₂-TiCl₄-VOCl₃ system, the vanadium removal mechanism is a synergistic reaction mechanism of reduction-complexation. It mainly forms polynuclear complexes with aluminum chloride, titanium chloride, and vanadyl chloride as central atoms bridged by Cl and O atoms in the reduction reaction. The polynuclear complexes undergo charge transfer and chemical reactions through the exchange and transfer of Cl and O atoms catalyzed by aluminum chloride (AlCl₃), removing VOCl₃ from crude titanium tetrachloride through two reaction pathways: one is that low-valent titanium (TiCl₃) converts VOCl₃ into insoluble VOCl₂; the other is that low-valent titanium (TiCl₂) converts VOCl₃ into insoluble VCl₃, with the generation of TiCl₂O. However, the finally generated AlCl₃ in the reaction system will convert TiCl₂O into TiCl₄, in which VOCl₃ is more easily converted into VCl₃.
+
+## Experimental Verification
+
+
+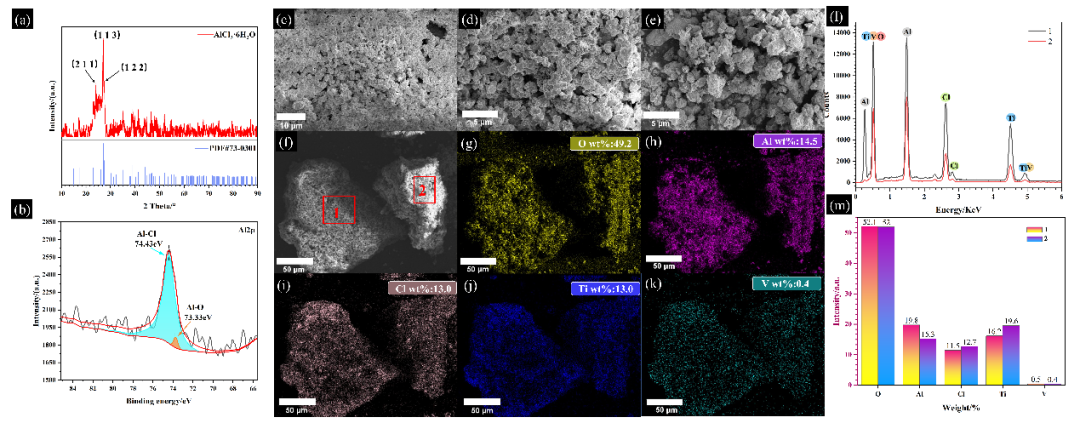 +
+*Fig. 2 (a) XRD image of residue, (b) XPS image of Al 2p, (c)~(k) SEM-EDS images of the residue. (c)~(f) SEM images of residue, (g)~(k) Overall EDS images of residue (f) map, (l)~(m) EDS images of specific regions of the residue (f) map*
+
+
+The XRD pattern of the residue is shown in Figure 2(a). There are three highest diffraction peaks near 24°, 27°, and 27.5°, corresponding to the (211), (113), and (122) crystal planes of AlCl₃·6H₂O, respectively. Combined with the Al 2p XPS spectrum in Figure 2(b), it is found that the residue mainly contains AlCl₃. The amount of AlCl₃ generated is consistent with the products in the thermodynamic calculation reaction equations R4 and R5, and AlCl₃ is observed to be generated in both reaction pathways in the DPMD simulation, indicating that AlCl₃ is generated by the reaction of Al in crude TiCl₄ and plays a certain catalytic role. The experiment further verifies the results of thermodynamic and DPMD simulations. At the same time, Figures 2(g)-(m) show the overall EDS images of Figure 2(f) and the specific collection areas 1 and 2, revealing the presence and uniform distribution of elements O, Al, Cl, Ti, and V, further confirming the presence of AlCl₃ and providing evidence for the presence of vanadium oxide, vanadium chloride, and titanium chloride.
+
+
+
+
+*Fig. 2 (a) XRD image of residue, (b) XPS image of Al 2p, (c)~(k) SEM-EDS images of the residue. (c)~(f) SEM images of residue, (g)~(k) Overall EDS images of residue (f) map, (l)~(m) EDS images of specific regions of the residue (f) map*
+
+
+The XRD pattern of the residue is shown in Figure 2(a). There are three highest diffraction peaks near 24°, 27°, and 27.5°, corresponding to the (211), (113), and (122) crystal planes of AlCl₃·6H₂O, respectively. Combined with the Al 2p XPS spectrum in Figure 2(b), it is found that the residue mainly contains AlCl₃. The amount of AlCl₃ generated is consistent with the products in the thermodynamic calculation reaction equations R4 and R5, and AlCl₃ is observed to be generated in both reaction pathways in the DPMD simulation, indicating that AlCl₃ is generated by the reaction of Al in crude TiCl₄ and plays a certain catalytic role. The experiment further verifies the results of thermodynamic and DPMD simulations. At the same time, Figures 2(g)-(m) show the overall EDS images of Figure 2(f) and the specific collection areas 1 and 2, revealing the presence and uniform distribution of elements O, Al, Cl, Ti, and V, further confirming the presence of AlCl₃ and providing evidence for the presence of vanadium oxide, vanadium chloride, and titanium chloride.
+
+
+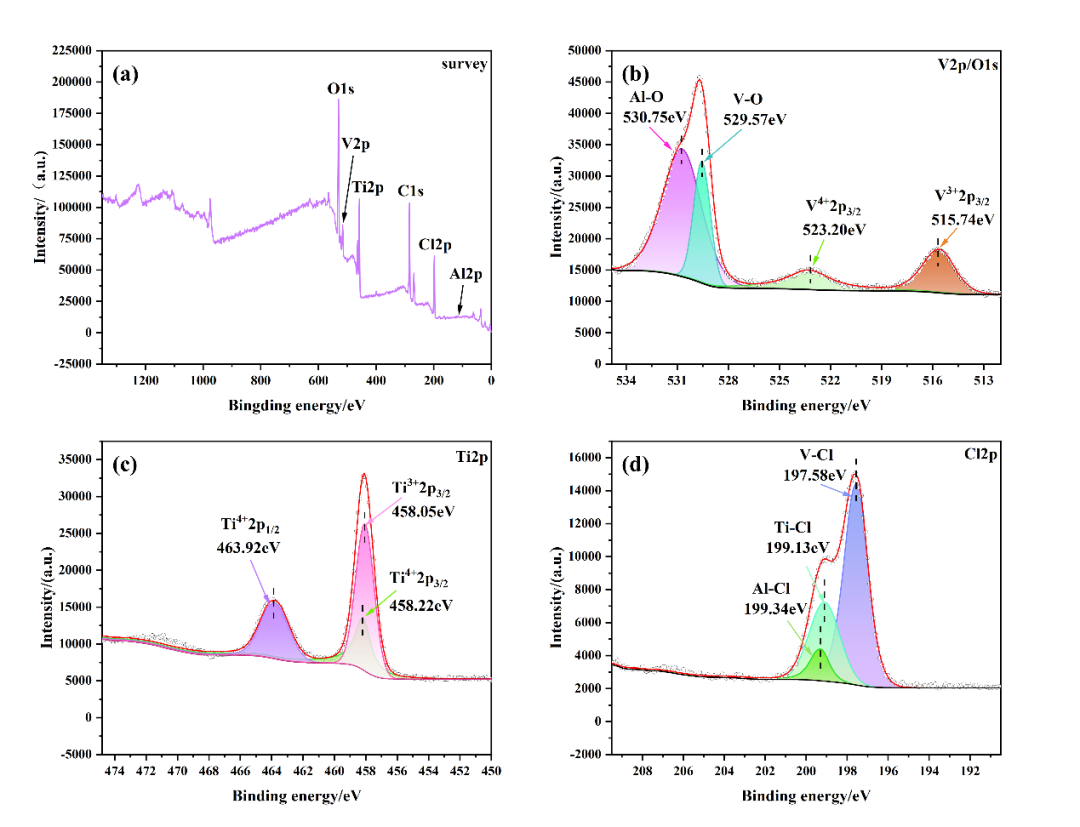 +
+ *Fig. 3 Residue XPS images: (a) XPS full spectrum of residue, (b) XPS image of V 2p/O 1s binding, (c) XPS image of Ti 2p, (d) XPS image of Cl 2p*
+
+
+The XPS characterization of the residue is shown in Figure 3. In Figure 3(a), the XPS full spectrum of the residue shows the elements V, Al, Cl, Ti, and O, which are consistent with the elements detected by EDS and the product elements observed in the DPMD simulation. In Figure 3(b), the XPS image of V 2p/O 1s binding shows that the valence states of V are +4 and +3, which form V-O and V-Cl bonds with O 1s and Cl 2p, indicating the presence of compounds such as VOCl₂ and VCl₃ and the products of the two reaction pathways simulated by DPMD, which confirms the thermodynamic calculation reaction equations R7 and R15. In addition, Al-O bonds are found at 530.75 eV in the V 2p/O 1s XPS spectrum, and Al-Cl bonds are found at 199.34 eV in the Cl 2p XPS spectrum (Figure 3(d)), indicating the possible presence of AlOCl, which confirms the reaction equation R15 obtained through thermodynamic calculation. In the XPS images of Ti 2p and Cl 2p shown in Figure 3(c), Ti³⁺, Ti⁴⁺2p3/2, and Ti⁴⁺2p1/2 are found at 458.05 eV, 458.22 eV, and 463.92 eV, indicating the presence of TiCl₃ and TiCl₄ in the residue. The analysis of chemical bonds and valence states in the above results is consistent with the DPMD simulation results, indicating that the surface residue contains VOCl₂, VCl₃, AlCl₃, and a small amount of residual TiCl₄, and V is mainly removed in the form of V⁴⁺ and V³⁺, further confirming the occurrence of reaction equations R7 and R15.
+
+
+
+
+ *Fig. 3 Residue XPS images: (a) XPS full spectrum of residue, (b) XPS image of V 2p/O 1s binding, (c) XPS image of Ti 2p, (d) XPS image of Cl 2p*
+
+
+The XPS characterization of the residue is shown in Figure 3. In Figure 3(a), the XPS full spectrum of the residue shows the elements V, Al, Cl, Ti, and O, which are consistent with the elements detected by EDS and the product elements observed in the DPMD simulation. In Figure 3(b), the XPS image of V 2p/O 1s binding shows that the valence states of V are +4 and +3, which form V-O and V-Cl bonds with O 1s and Cl 2p, indicating the presence of compounds such as VOCl₂ and VCl₃ and the products of the two reaction pathways simulated by DPMD, which confirms the thermodynamic calculation reaction equations R7 and R15. In addition, Al-O bonds are found at 530.75 eV in the V 2p/O 1s XPS spectrum, and Al-Cl bonds are found at 199.34 eV in the Cl 2p XPS spectrum (Figure 3(d)), indicating the possible presence of AlOCl, which confirms the reaction equation R15 obtained through thermodynamic calculation. In the XPS images of Ti 2p and Cl 2p shown in Figure 3(c), Ti³⁺, Ti⁴⁺2p3/2, and Ti⁴⁺2p1/2 are found at 458.05 eV, 458.22 eV, and 463.92 eV, indicating the presence of TiCl₃ and TiCl₄ in the residue. The analysis of chemical bonds and valence states in the above results is consistent with the DPMD simulation results, indicating that the surface residue contains VOCl₂, VCl₃, AlCl₃, and a small amount of residual TiCl₄, and V is mainly removed in the form of V⁴⁺ and V³⁺, further confirming the occurrence of reaction equations R7 and R15.
+
+
+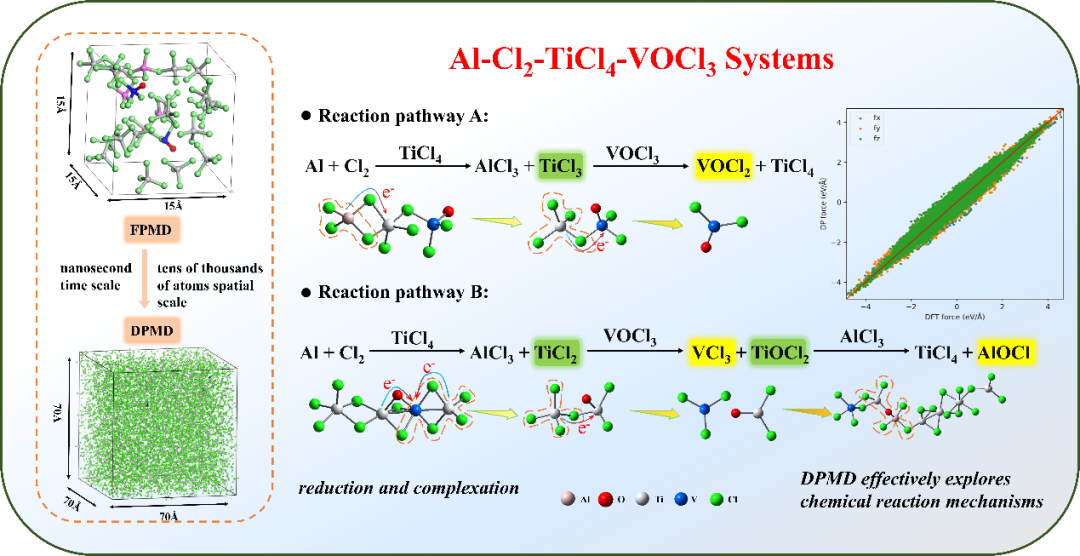 +
+*Fig 4. Two vanadium removal reaction pathways in aluminum vanadium removal*
+
+
+Conclusion:This study shows through thermodynamic calculation results that there are two different reaction pathways for vanadium removal by aluminum: using generated low-valent titanium (TiCl₂, TiCl₃) to convert VOCl₃ into vanadium chloride (VCl₃, VCl₄(g)) or vanadyl chloride (VOCl, VOCl₂), as shown in Figure 4. DPMD simulations show that the vanadium removal reaction in the Al-Cl₂-TiCl₄-VOCl₃ system is a synergistic mechanism of reduction-complexation reactions. In the reduction process, polynuclear complexes composed of aluminum, titanium, and vanadium as central atoms bridged by Cl and O atoms are formed. Catalyzed by aluminum chloride, chemical reduction reactions occur through the exchange and transfer of Cl and O atoms to transfer charges, determining two reaction pathways for low-valent titanium (TiCl₂, TiCl₃) to convert VOCl₃ into VOCl₂ and VCl₃. According to theoretical and simulation calculations, experimental research on vanadium removal by adding aluminum powder was carried out. The phase analysis, micro morphology, and chemical valence state of the residue were detected. The results show that the residue mainly contains amorphous AlCl₃·6H₂O, the valence states of Ti are +3 and +4, and V³⁺ and V⁴⁺ exist, confirming the presence of VCl₃ and VOCl₂. The experimental research further verifies the theoretical calculation results. It can be seen that the DPMD simulation method is effective in studying chemical reaction mechanisms at the nanosecond time scale (4 ns) and the spatial scale of tens of thousands of atoms. It can not only accurately describe the reaction dynamics behavior of the system but also efficiently simulate the chemical reaction process of complex multi-component systems, providing a reliable calculation method for studying the vanadium removal reaction system by aluminum. The DPMD method has considerable application potential in helping solve complex reaction problems in industrial fields and promoting the development of molecular science mechanism research.
+
+
+*Fig 4. Two vanadium removal reaction pathways in aluminum vanadium removal*
+
+
+Conclusion:This study shows through thermodynamic calculation results that there are two different reaction pathways for vanadium removal by aluminum: using generated low-valent titanium (TiCl₂, TiCl₃) to convert VOCl₃ into vanadium chloride (VCl₃, VCl₄(g)) or vanadyl chloride (VOCl, VOCl₂), as shown in Figure 4. DPMD simulations show that the vanadium removal reaction in the Al-Cl₂-TiCl₄-VOCl₃ system is a synergistic mechanism of reduction-complexation reactions. In the reduction process, polynuclear complexes composed of aluminum, titanium, and vanadium as central atoms bridged by Cl and O atoms are formed. Catalyzed by aluminum chloride, chemical reduction reactions occur through the exchange and transfer of Cl and O atoms to transfer charges, determining two reaction pathways for low-valent titanium (TiCl₂, TiCl₃) to convert VOCl₃ into VOCl₂ and VCl₃. According to theoretical and simulation calculations, experimental research on vanadium removal by adding aluminum powder was carried out. The phase analysis, micro morphology, and chemical valence state of the residue were detected. The results show that the residue mainly contains amorphous AlCl₃·6H₂O, the valence states of Ti are +3 and +4, and V³⁺ and V⁴⁺ exist, confirming the presence of VCl₃ and VOCl₂. The experimental research further verifies the theoretical calculation results. It can be seen that the DPMD simulation method is effective in studying chemical reaction mechanisms at the nanosecond time scale (4 ns) and the spatial scale of tens of thousands of atoms. It can not only accurately describe the reaction dynamics behavior of the system but also efficiently simulate the chemical reaction process of complex multi-component systems, providing a reliable calculation method for studying the vanadium removal reaction system by aluminum. The DPMD method has considerable application potential in helping solve complex reaction problems in industrial fields and promoting the development of molecular science mechanism research.
+
 +
+*Fig. 1 (a), (b) the microstructure evolution images of two reaction pathways of VOCl3 in the 413K Al-Cl2-TiCl4-VOCl3 system from DPMD; (c) Fig. (a) RDF image of different atoms around V at 200 ps; (d) Fig. (b) RDF image of different atoms around Al and between Ti-O at 400 ps*
+
+
+*Fig. 1 (a), (b) the microstructure evolution images of two reaction pathways of VOCl3 in the 413K Al-Cl2-TiCl4-VOCl3 system from DPMD; (c) Fig. (a) RDF image of different atoms around V at 200 ps; (d) Fig. (b) RDF image of different atoms around Al and between Ti-O at 400 ps*
+ +
+*Fig. 2 (a) XRD image of residue, (b) XPS image of Al 2p, (c)~(k) SEM-EDS images of the residue. (c)~(f) SEM images of residue, (g)~(k) Overall EDS images of residue (f) map, (l)~(m) EDS images of specific regions of the residue (f) map*
+
+
+*Fig. 2 (a) XRD image of residue, (b) XPS image of Al 2p, (c)~(k) SEM-EDS images of the residue. (c)~(f) SEM images of residue, (g)~(k) Overall EDS images of residue (f) map, (l)~(m) EDS images of specific regions of the residue (f) map*
+ +
+ *Fig. 3 Residue XPS images: (a) XPS full spectrum of residue, (b) XPS image of V 2p/O 1s binding, (c) XPS image of Ti 2p, (d) XPS image of Cl 2p*
+
+
+ *Fig. 3 Residue XPS images: (a) XPS full spectrum of residue, (b) XPS image of V 2p/O 1s binding, (c) XPS image of Ti 2p, (d) XPS image of Cl 2p*
+ +
+*Fig 4. Two vanadium removal reaction pathways in aluminum vanadium removal*
+
+
+*Fig 4. Two vanadium removal reaction pathways in aluminum vanadium removal*
+